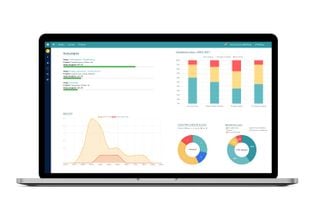
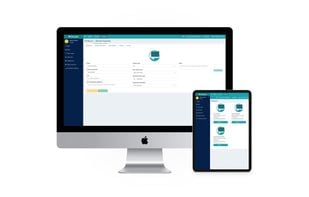
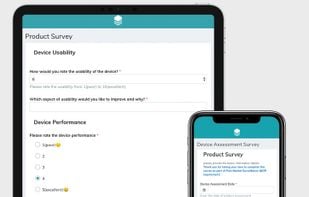
EDC solution for simple, secure, and cost-efficient clinical data capture for the validation and Post-Market Surveillance (PMS / PMCF) of medical devices in line with the EU MDR. Features: ePRO, eCOA, eDiaries, eSurveys and more. Made in Germany.
Cost / License
- Paid
- Proprietary
Platforms
- Online
- Software as a Service (SaaS)